Streamlined Compliance Support
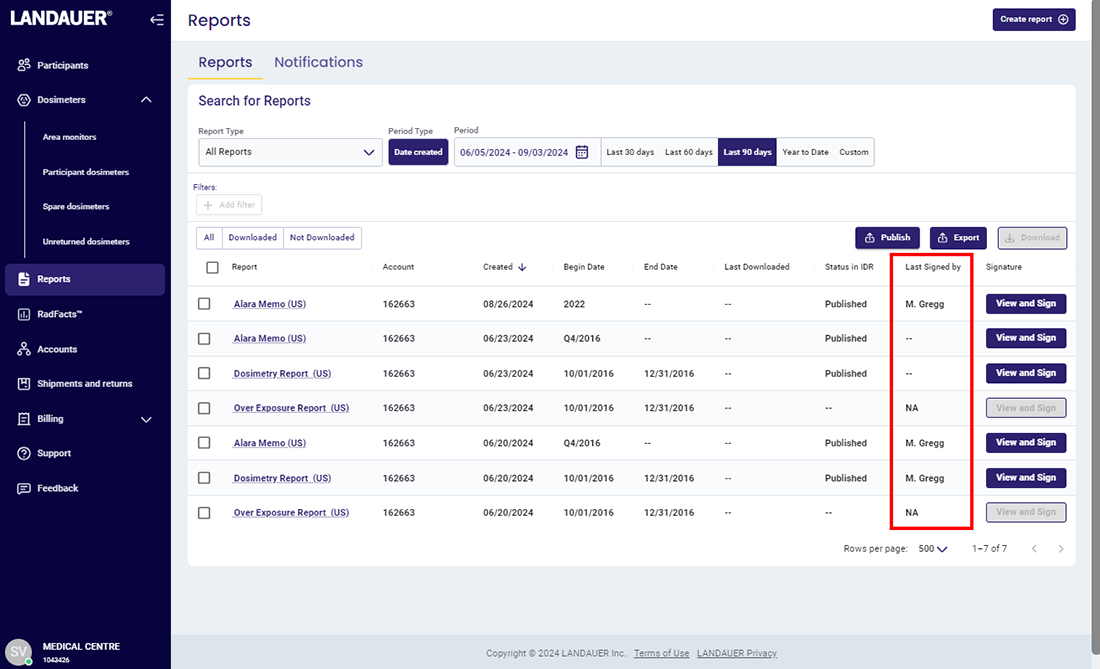
Signature Suite™ is a compliance documentation upgrade available for myLDR that simplifies record-keeping for compliance purposes, reduces manual activities around reporting, and saves valuable time!
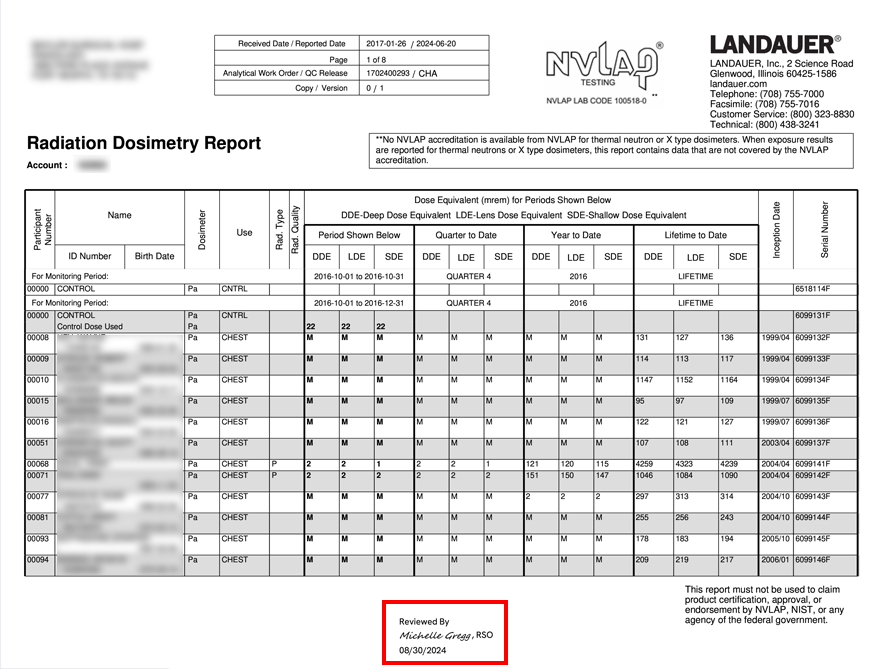
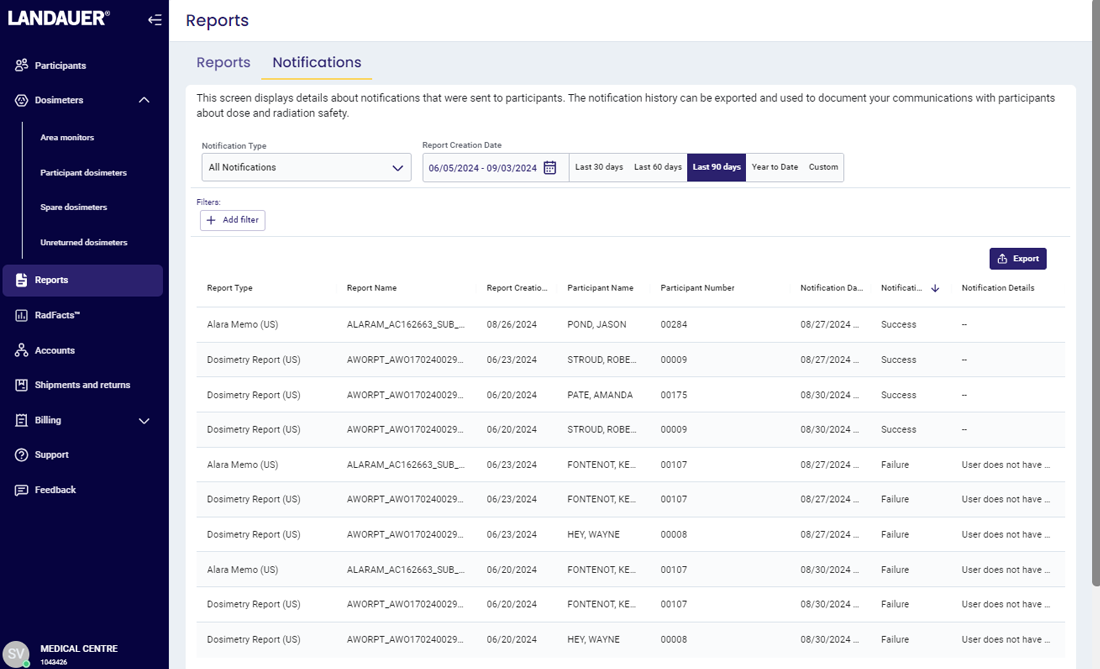
For an NRC or similar inspection, organizations are required to provide inspectors with documentation that proves 1) they are reviewing dosimetry reports and 2) the reports are being provided to their participants.
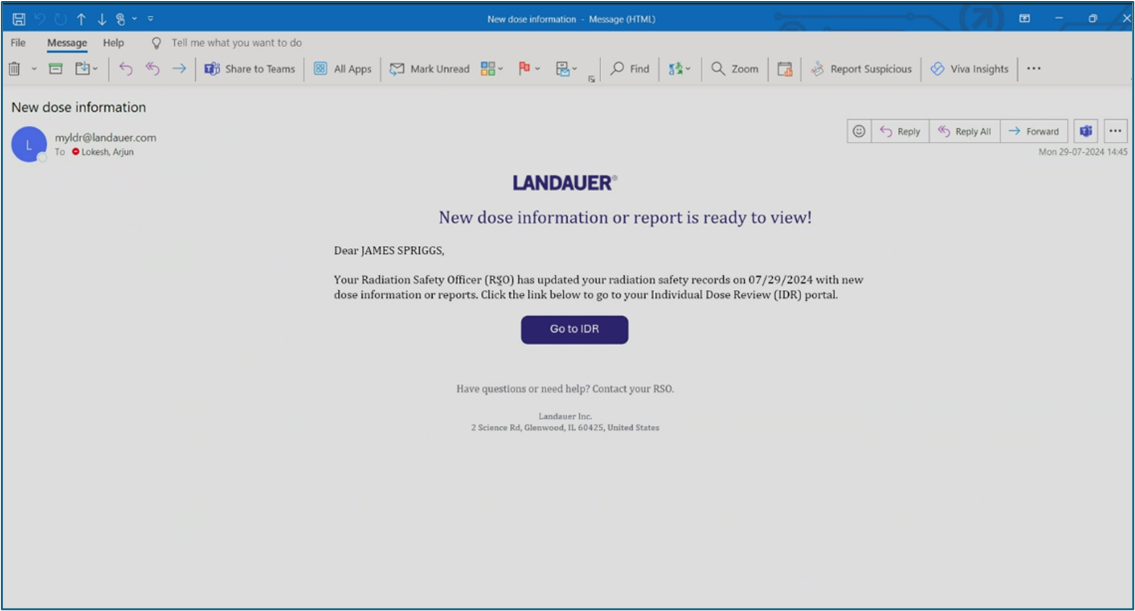
Many organizations are using cumbersome, manual processes such as requiring employees to sign paper documents, scanning reports and records, sending individual emails and saving history for many years, and storing documentation using outdated methods.
myLDR Signature Suite reduces the time, risk, and effort involved with meeting compliance requirements by enabling subscribers to leverage a completely digital workflow!